
A Comprehensive Guide to Toxicology in Nonclinical Drug Development
1.948 kr.
1.948 kr.
Tidligere laveste pris:
1.973 kr.
Fre., 18 juli - tors., 24 juli
Sikker betaling
14 dages åbent køb
Sælges og leveres af
AdlibrisProduktbeskrivelse
Varenr.
2a677a4d-a27c-4af0-a9dd-478da59d8a57
A Comprehensive Guide to Toxicology in Nonclinical Drug Development
1.948 kr.
1.948 kr.
Tidligere laveste pris:
1.973 kr.
Fre., 18 juli - tors., 24 juli
Sikker betaling
14 dages åbent køb
Sælges og leveres af
AdlibrisLignende topsælgere
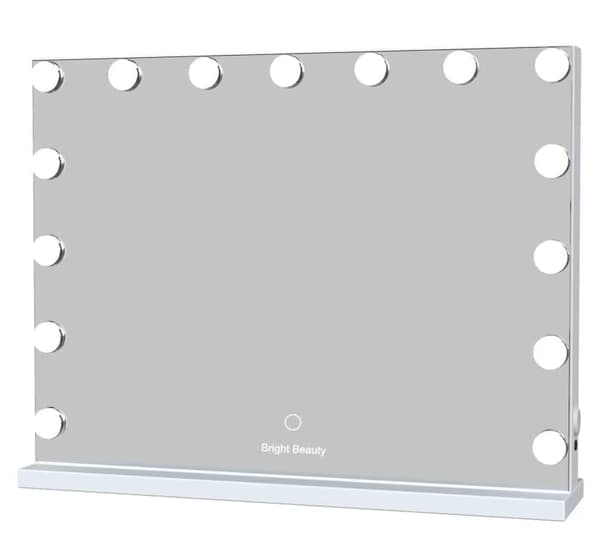
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
539 kr.
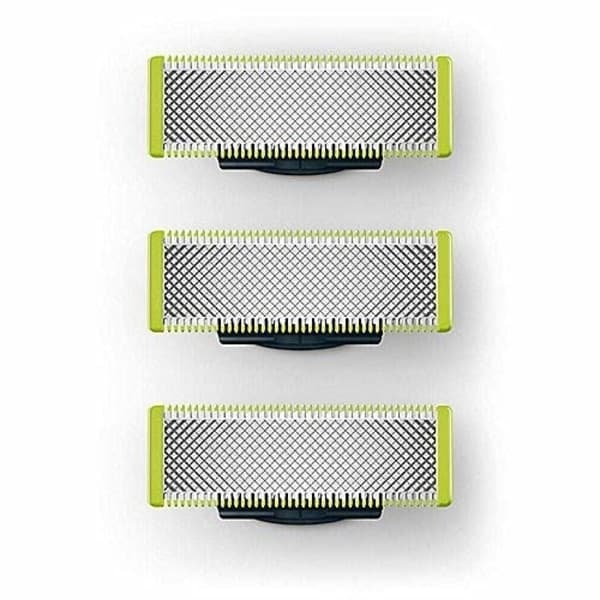
3-pak barberblade, der er kompatible med Philips Oneblade Replacement
106 kr.

POP MART Labubu The Monsters Big Into Energy Series Figures Vinyl Plush Pendant Blind Box
439 kr.

OBOSOE trådløs CarPlay-adapter, til CarPlay og Android Auto, Plug-and-play med ultralav latenstid
190 kr.
Tidligere laveste pris:
266 kr.

OBOSOE Fleksibel klapvognsventilator – Bærbar ventilator med batteri, genopladelig med 3 hastigheder, til klapvogn, seng eller kontor Sort
86 kr.
Tidligere laveste pris:
116 kr.

POP MART Labubu The Monsters Exciting Macaron Vinyl Face Blind Box
999 kr.

G4 Halogenpærer / Stiftpærer - Halogen 10W (10-Pack)
69 kr.

Montessori Busy Board til børn 1-4 år, 12-sidet aktivitetslegetøj med Reißverschluss
140 kr.

FENCHILIIN Stor Hollywood Makeup Spejl med lys USB bordplade vægbeslag hvid 80 x 58 cm
961 kr.

Timer til Klasselokalet - 60 minutter
149 kr.
Anbefalede produkter

FENCHILIIN hollywood spejl makeup spejl med lys dæmpbar med tre lystilstande bordplade vægbeslag make up spejl med belysning hvid 58 x 46cm
621 kr.

2-Pak - Lader til iPhone - Hurtiglader - Adapter + Kabel 20W USB-C
139 kr.

Wii til HDMI Adapter 1080p Full-HD kompatibel til Nintendo
49 kr.

Ørepuder til Bose QuietComfort - QC35/QC25/QC15/AE2 Hovedtelefoner Grå
69 kr.

3-Pak - Fidget Spinners med Sugekop til Børn
119 kr.

INF Ørepuder til Bose QC35 I/II, QC25, QC15, QC 2 AE 2, AE 2i, AE 2w, SoundTrue, SoundLink Sort
69 kr.
Tidligere laveste pris:
89 kr.

20-pak G4 halogenlamper 20W 12V - Varm hvid
99 kr.

OBOSOE bærbar håndblæser/blæser med turbokraft – 5-trins, 3-i-1-funktion, perfekt til rejser og udendørsaktiviteter, kan hænges på nakken
114 kr.

Tabel ben tabelramme trapezformet designe industrielt design bordfod 60 x 72 cm stål
577 kr.

FENCHILIIN Hollywood stort makeup spejl med lamper bordplade vægmonteret hvid spejl med lys
511 kr.