
Medical Regulatory Affairs
2.160 kr.
2.160 kr.
Tors., 15 maj - ons., 21 maj
Sikker betaling
14 dages åbent køb
Sælges og leveres af
Adlibris
Produktbeskrivelse
This handbook covers medical device regulatory systems in different countries, ISO standards for medical devices, clinical trial and regulatory requirements, and documentation for application. It is the first to cover the medical device regulatory affairs in Asia. Experts from influential international regulatory bodies, including the US Food and Drug Administration (FDA), UK Medicines and Healthcare Products Regulatory Agency, Japan Pharmaceuticals and Medical Devices Agency, Saudi Food and Drug Authority, Korea Testing Laboratory, Taiwan FDA, World Health Organization, Asian Harmonization Working Party, Regulatory Affairs Professionals Society, and British Standards Institution, have contributed to the book. Government bodies, the medical device industry, academics, students, and general readers will find the book immensely useful for understanding the global regulatory environment and in their research and development projects.
Varenr.
f40b79ed-7ec9-4ce5-b915-635d914e204d
Medical Regulatory Affairs
2.160 kr.
2.160 kr.
Tors., 15 maj - ons., 21 maj
Sikker betaling
14 dages åbent køb
Sælges og leveres af
Adlibris
Lignende topsælgere
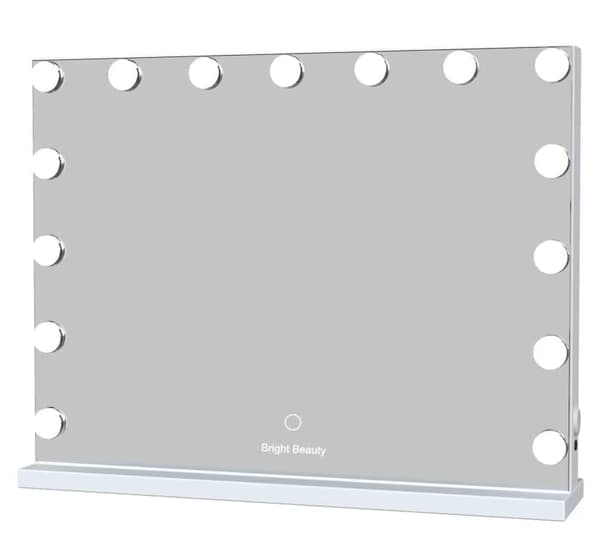
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
539 kr.

Timer til Klasselokalet - 60 minutter
169 kr.

Silikonetape / Plaster til Ar – Behandling af ar
69 kr.

FENCHILIIN Hollywood stort makeup spejl med lamper bordplade vægmonteret hvid spejl med lys
479 kr.
Tidligere laveste pris:
539 kr.

FENCHILIIN hollywood spejl makeup spejl med lys dæmpbar med tre lystilstande bordplade vægbeslag make up spejl med belysning hvid 58 x 46cm
621 kr.

FENCHILIIN Hollywood makeup spejl med lys forstørrelse 360° drejelig bordplade hvid spejl 30 x 41 cm
279 kr.
Tidligere laveste pris:
331 kr.

FENCHILIIN Stor Hollywood Makeup Spejl med lys Bluetooth Krystal Finish Bordplade Vægbeslag Hvid 80 x 58 cm Spejl
521 kr.
Tidligere laveste pris:
795 kr.

94 CM 3 X Plæneklipperknive til Husqvarna RIDER R213 R214 R215 R216 R316 R318 R320 AWD
265 kr.

FENCHILIIN Hollywood Makeup Spejl Med Lys 360° drejelig dæmpbar med tre lystilstande bordplade Spejl hvid 65 x 49 cm
439 kr.
Tidligere laveste pris:
521 kr.

G4 Halogenpærer / Stiftpærer - Halogen 10W (10-Pack)
69 kr.
Anbefalede produkter

10 stk Biodance Bio-Collagen Real Deep Mask – Collagen Sheet Face Overnight Mask
259 kr.

Ørepuder til Bose QuietComfort - QC35/QC25/QC15/AE2 Hovedtelefoner Svart
69 kr.

RCA AV till HDMI Converter / Adapter 1080P Universal White
79 kr.

FENCHILIIN Stor Hollywood Makeup Spejl med lys USB bordplade vægbeslag hvid 80 x 58 cm
961 kr.

Lader til iPhone - Hurtiglader - Adapter + Kabel 20W USB-C 3-Pack iPhone
179 kr.

4 X 17CM plæneklipper kniv til Stiga Ready Park Villa 85 95 105 Combi 1134-7880-01 1134-9119-01
266 kr.

INF 12-pak trådspoler med 3 betræk til Ryobi græstrimmere MultiColor
164 kr.

3-pak AGA AQVIA PET-flaske, 1L (hvid)
159 kr.

20-pak G4 halogenlamper 20W 12V - Varm hvid
99 kr.

VONROC Teleskop - rengørings - børste - 6,50m | Inkl. multibørste, 2-i-1 vinduesvasker, slange og sæbedispenser
489 kr.