Encyclopedia of Biopharmaceutical Statistics - Four Volume Set
13.662 kr.
13.662 kr.
Tidligere laveste pris:
13.844 kr.
Man., 21 juli - tors., 24 juli
Sikker betaling
14 dages åbent køb
Sælges og leveres af
AdlibrisProduktbeskrivelse
Since the publication of the first edition in 2000, there has been an explosive growth of literature in biopharmaceutical research and development of new medicines. This encyclopedia (1) provides a comprehensive and unified presentation of designs and analyses used at different stages of the drug development process, (2) gives a well-balanced summary of current regulatory requirements, and (3) describes recently developed statistical methods in the pharmaceutical sciences.
Features of the Fourth Edition:
1. 78 new and revised entries have been added for a total of 308 chapters and a fourth volume has been added to encompass the increased number of chapters.
2. Revised and updated entries reflect changes and recent developments in regulatory requirements for the drug review/approval process and statistical designs and methodologies.
3. Additional topics include multiple-stage adaptive trial design in clinical research, translational medicine, design and analysis of biosimilar drug development, big data analytics, and real world evidence for clinical research and development.
4. A table of contents organized by stages of biopharmaceutical development provides easy access to relevant topics.
About the Editor:
Shein-Chung Chow, Ph.D. is currently an Associate Director, Office of Biostatistics, U.S. Food and Drug Administration (FDA). Dr. Chow is an Adjunct Professor at Duke University School of Medicine, as well as Adjunct Professor at Duke-NUS, Singapore and North Carolina State University. Dr. Chow is the Editor-in-Chief of the Journal of Biopharmaceutical Statistics and the Chapman & Hall/CRC Biostatistics Book Series and the author of 28 books and over 300 methodology papers. He was elected Fellow of the American Statistical Association in 1995.
Varenr.
53a05c9a-237b-4044-8ed8-6a578a4a0d32
Encyclopedia of Biopharmaceutical Statistics - Four Volume Set
13.662 kr.
13.662 kr.
Tidligere laveste pris:
13.844 kr.
Man., 21 juli - tors., 24 juli
Sikker betaling
14 dages åbent køb
Sælges og leveres af
AdlibrisLignende topsælgere
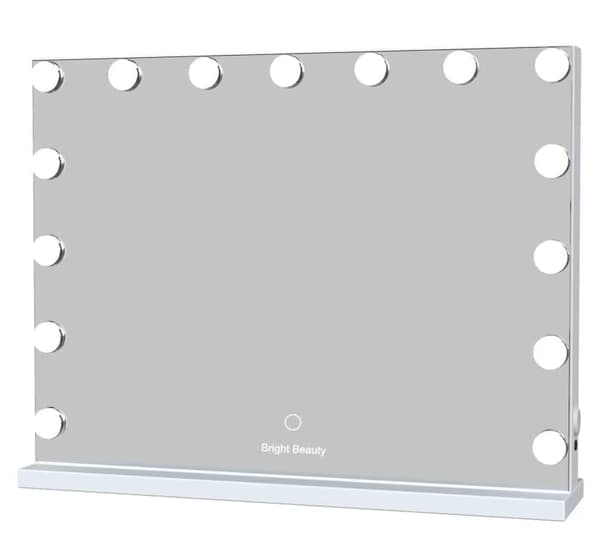
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
539 kr.
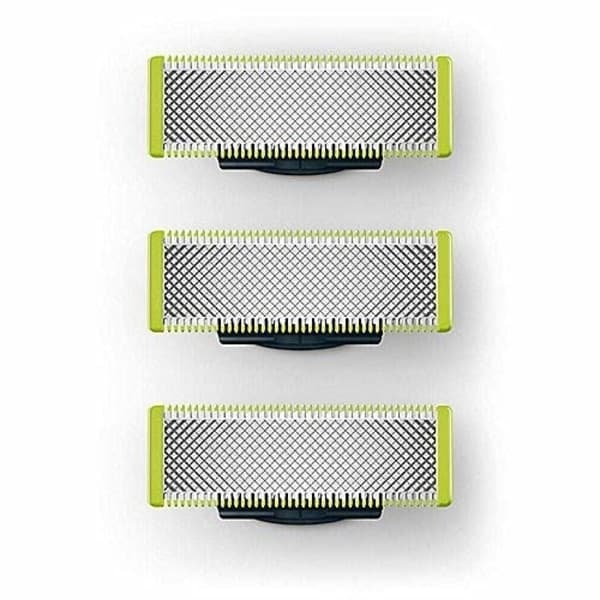
3-pak barberblade, der er kompatible med Philips Oneblade Replacement
106 kr.

RCA AV till HDMI Converter / Adapter 1080P Universal White
79 kr.

Saphe One+ hastigheds- og trafikalarm
468 kr.

POP MART Labubu The Monsters Big Into Energy Series Figures Vinyl Plush Pendant Blind Box
439 kr.

OBOSOE trådløs CarPlay-adapter, til CarPlay og Android Auto, Plug-and-play med ultralav latenstid
190 kr.
Tidligere laveste pris:
266 kr.

OBOSOE Fleksibel klapvognsventilator – Bærbar ventilator med batteri, genopladelig med 3 hastigheder, til klapvogn, seng eller kontor Sort
86 kr.
Tidligere laveste pris:
116 kr.

G4 Halogenpærer / Stiftpærer - Halogen 10W (10-Pack)
69 kr.

POP MART Labubu The Monsters Exciting Macaron Vinyl Face Blind Box
799 kr.

Timer til Klasselokalet - 60 minutter
149 kr.
Anbefalede produkter

LED-belysning og 4 skuffer, dobbeltseng Opbevaringssenge Sengeramme, trælameller (uden madras) Beige 180x200cm
3.378 kr.

FENCHILIIN hollywood spejl makeup spejl med lys dæmpbar med tre lystilstande bordplade vægbeslag make up spejl med belysning hvid 58 x 46cm
621 kr.

2-Pak - Lader til iPhone - Hurtiglader - Adapter + Kabel 20W USB-C
139 kr.

Montessori Busy Board til børn 1-4 år, 12-sidet aktivitetslegetøj med Reißverschluss
140 kr.

FENCHILIIN Stor Hollywood Makeup Spejl med lys USB bordplade vægbeslag hvid 80 x 58 cm
961 kr.

Wii til HDMI Adapter 1080p Full-HD kompatibel til Nintendo
49 kr.

FENCHILIIN Hollywood makeup spejl med lys forstørrelse 360° drejelig bordplade hvid spejl 30 x 41 cm
395 kr.

3-Pak - Fidget Spinners med Sugekop til Børn
119 kr.

20-pak G4 halogenlamper 20W 12V - Varm hvid
99 kr.

3-delt sæt dobbeltsidet skærebræt i rustfrit stål, skimmel- og fugtbestandigt
295 kr.