
Biocompatibility Protocols for Medical Devices and Materials
1.386 kr.
1.386 kr.
Man., 30 juni - fre., 4 juli
Sikker betaling
14 dages åbent køb
Sælges og leveres af
AdlibrisProduktbeskrivelse
Varenr.
9e68359c-efb8-40ff-8a8b-f82712814f6e
Biocompatibility Protocols for Medical Devices and Materials
1.386 kr.
1.386 kr.
Man., 30 juni - fre., 4 juli
Sikker betaling
14 dages åbent køb
Sælges og leveres af
AdlibrisLignende topsælgere
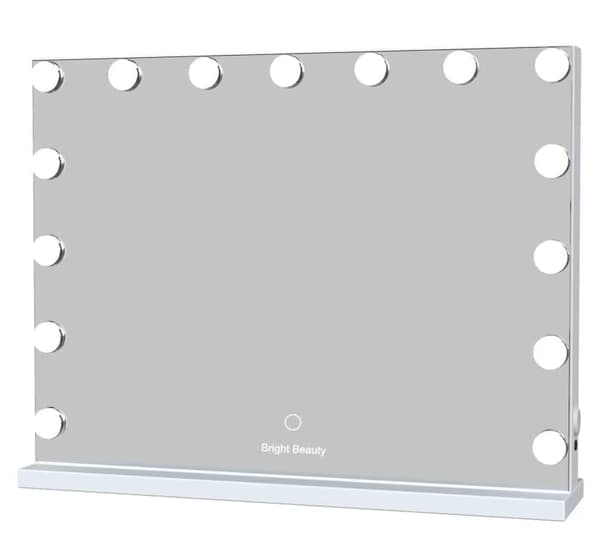
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
539 kr.

POP MART Labubu Monsters Macaron Vinyl Face 17cm Blind Box
122 kr.
Tidligere laveste pris:
123 kr.

POP MART Labubu The Monsters – Exciting Macaron Blind Box 17 cm Vinyl Samlerfigur | Designer Toy | Original produkt
169 kr.

1 stk. POP MART Labubu 3.0 Big into Energy Blind Box Figur – The Monsters Vinyl Plysch Hänge 17 cm (tilfældig farve, enkelt pakke)
169 kr.

POP MART Labubu The Monsters – Big into Energy Series Blind Box 17 cm Vinyl Samlerfigur | Designer Toy | Original produkt
159 kr.

Montessori Busy Board til børn 1-4 år, 12-sidet aktivitetslegetøj med Reißverschluss
140 kr.

Trådløs CarPlay-adapter 2025, til CarPlay og Android Auto, Plug-and-Play
155 kr.

Timer til Klasselokalet - 60 minutter
149 kr.

1 stk POP MART Labubu 2.0 The Monsters Macaron Blind Box Plysfigur (tilfældig farve, 17 cm, generation 2, 1-pakke)
169 kr.

Ørepuder til Bose QuietComfort - QC35/QC25/QC15/AE2 Hovedtelefoner Svart
69 kr.
Anbefalede produkter

Secret Hitler Brætspil - strategispil Puslespil for 2-8 personer
276 kr.

FENCHILIIN hollywood spejl makeup spejl med lys dæmpbar med tre lystilstande bordplade vægbeslag make up spejl med belysning hvid 58 x 46cm
621 kr.

G4 Halogenpærer / Stiftpærer - Halogen 10W (10-Pack)
69 kr.

94 CM 3 X Plæneklipperknive til Husqvarna RIDER R213 R214 R215 R216 R316 R318 R320 AWD
265 kr.

FENCHILIIN Hollywood stort makeup spejl med lamper bordplade vægmonteret hvid spejl med lys
479 kr.

FENCHILIIN Hollywood makeup spejl med lys forstørrelse 360° drejelig bordplade hvid spejl 30 x 41 cm
359 kr.

Hurtigoplader Garmin Ure - Universal Oplader
49 kr.

20-pak G4 halogenlamper 20W 12V - Varm hvid
99 kr.

FENCHILIIN Stor Hollywood Makeup Spejl med lys USB bordplade vægbeslag hvid 80 x 58 cm
961 kr.

XXL-hængekøje med stålstel op til 250 kg Beige inkl. bæretaske ML-Design
621 kr.