
Planning, Writing and Reviewing Medical Device Clinical and Performance Evaluation Reports (CERs/PERs)
1.296 kr.
1.296 kr.
På lager
Tors., 6 feb. - ons., 12 feb.
Sikker betaling
14 dages åbent køb
Sælges og leveres af
Adlibris
Produktbeskrivelse
A Practical Guide to Planning, Writing, and Reviewing Medical Device Clinical Evaluation Reports guides readers through clinical data evaluation of medical devices that is in compliance with the EU MDR requirements and other similar regulatory requirements throughout the world. This book brings together knowledge learned as the author constructed hundreds of CERs and taught thousands of learners on how to conduct clinical data evaluations. This book will support training for clinical engineers, clinical evaluation scientists, and experts reviewing medical device CERs, and will help individual writers, teams and companies to develop stronger, more robust CERs.
Varenr.
47ffc36e-6ec9-56c2-8bbd-1887f5a32732
Planning, Writing and Reviewing Medical Device Clinical and Performance Evaluation Reports (CERs/PERs)
1.296 kr.
1.296 kr.
På lager
Tors., 6 feb. - ons., 12 feb.
Sikker betaling
14 dages åbent køb
Sælges og leveres af
Adlibris
Lignende topsælgere

Bright Beauty
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
599 kr.
4,5(72)
onsdag, 15 jan.
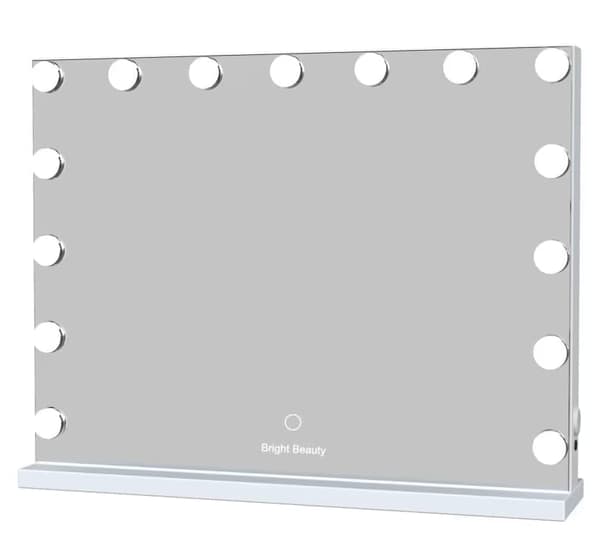
Bright Beauty Vanity
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
659 kr.
4,3(897)
onsdag, 15 jan.

Bigzzia
2-i-1 foldeløbebånd, elektrisk løbebånd under skrivebordet, jogging til hjemmekontoret Sort
1.549 kr.
4,2(295)
torsdag, 23 jan.

Generic
Timer til Klasselokalet - 60 minutter
169 kr.
4,2(55)
onsdag, 22 jan.

Bright Beauty Vanity
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande - roterbar
549 kr.
4,1(45)
onsdag, 15 jan.

Fenchilin
FENCHILIIN Stor Hollywood Makeup Spejl med lys USB bordplade vægbeslag hvid 80 x 58 cm
961 kr.
4,6(165)
onsdag, 15 jan.

A Series
RCA AV till HDMI Converter / Adapter 1080P Universal White
79 kr.
Tidligere laveste pris:
99 kr.
5,0(2)
tirsdag, 14 jan.

Best Trade
Nintendo Wii til HDMI Adapter 1080p Full-HD
47 kr.
4,4(37)
fredag, 24 jan.

Rattrix
Sammenfoldelig justerbar vægtbænk, 660 LB kapacitet - Kompakt og sammenfoldelig vægtbænk til hjemmegymnastik, fuld kropstræning B
549 kr.
5,0(1)
onsdag, 22 jan.

Vitu
Nødradio med solceller og håndsving Solcelle 2000mAh Powerbank SOS
199 kr.
4,2(117)
onsdag, 15 jan.
Anbefalede produkter

Fenchilin
FENCHILIIN Hollywood Makeup Spejl Med Lys dæmpbar med tre lystilstande Bordplade vægbeslag hvid 58 x 46cm
695 kr.
4,6(192)
onsdag, 15 jan.

Fenchilin
FENCHILIIN Stor Hollywood Makeup Spejl med Lamper Bluetooth Table Top Vægbeslag Hvid 80 x 58 cm
932 kr.
4,6(72)
onsdag, 15 jan.

Remote TV
Ersättningsfjärrkontroll för Chromecast Google TV G9N9N
89 kr.
Tidligere laveste pris:
99 kr.
3,0(6)
mandag, 20 jan.

Bright Beauty Vanity
Bright Beauty Vanity Namira - make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
599 kr.
4,3(23)
onsdag, 15 jan.

Otego
Samsung Oplader Hurtigoplader - Adapter + Kabel 25W USB-C 2m
80 kr.
4,5(10)
onsdag, 22 jan.

Bright Beauty Vanity
Bright Beauty Vanity Nina - makeup spejl -make up spejl med belysning - hollywood spejl - schminke spejl med lys - hvid - dæmpbar med tre lystilstande
439 kr.
4,0(238)
onsdag, 15 jan.

Generic
Knivssæt til Børn 6-dele - Børnevenlige knive
129 kr.
4,3(25)
onsdag, 22 jan.

Generic
Ørepuder til Bose QuietComfort - QC35/QC25/QC15/AE2 Hovedtelefoner Svart
69 kr.
4,5(710)
onsdag, 22 jan.

Magnetic
Magnetic Tiles Kuglebane 88 dele
329 kr.
Tidligere laveste pris:
480 kr.
4,8(4)
onsdag, 15 jan.

Rivanoo
Mini trådløs CarPlay & Android Auto-adapter til iPhone & Android – Nyd friheden fra kabler!
349 kr.
Tidligere laveste pris:
399 kr.
5,0(2)
torsdag, 16 jan.